Midway Point & Keytruda Sticker Shock
This week marks the halfway point of my chemo/immunotherapy treatment plan. I have finished the third cycle of treatment and started the fourth of six planned cycles. I’m still feeling mostly normal, except for the expected days of fatigue and nausea around days 3-5 of a cycle. My oncologist did mention that the impact of chemo/immuno will be cumulative. However, I haven’t noticed much impact so far, and hopefully the falloff for the remaining cycles won’t be severe.
Overall, treatment is going well and I’m tolerating things well. I may even be feeling more optimistic than before, as there continues to be more research showing how effective immunotherapy can be. Hopefully my tumor will continue to respond to the drug combination I’m receiving.
For better or worse, I’ve become fairly adept at is understanding medical costs, claims, EOBs, deducible/out-of-pocket max, co-pays, billing, and other aspects of the business of healthcare. Even though I knew that we’d reach our out of pocket (OOP) max quickly this year, I still track how each service is billed and whether we’ll have financial responsibility. Now that I’ve seen a lot of claims, EOBs and bills (and had a lot of phone calls to resolve disputes), not much surprises me anymore. However, the billing for Keytruda (pembrolizumab), the immunotherapy drug, did cause me to take a closer look. (BTW, our current insurance is excellent, in terms of premium, costs, coverage and claims handling - much better than the last insurer.)
When I first saw the amount billed for Keytruda, I got a bit of sticker shock and thought it was the aggregate amount for all 6 cycles, not a per treatment amount. Since this is a drug that I’ll likely be taking long-term, possibly for the rest of my life, I don’t want it to be cost prohibitive. University of Chicago Medicine is billing Aetna over $67,000 per treatment (200mg for a 3 week cycle). Aetna’s negotiated rate (after plan discount) with UCM is $22,000. Of course, this is well above our OOP, so insurance will cover 100%. So we won’t have to worry about the cost the rest of this plan year, but it does mean that we’ll be paying the insurance OOP max every year I’ll be taking this.
UCM billing $67,451 for each Keytruda (pembrolizumab) infusion
Aetna’s negotiated rate, after plan discount is $22,461.29(!). They are now covering 100% after meeting out OOP
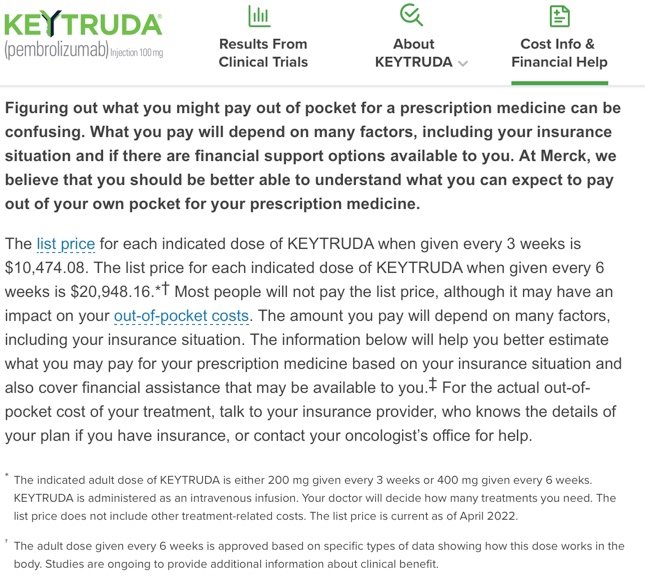
The interesting thing is that Keytruda’s own website shows that the “list price” is $10,000, so UCM is billing nearly 6.5x the list price! No wonder Keytruda there are so many TV ads! Joking aside, immunotherapy drugs are amazing at precisely targeting cancer cells and allowing my body’s own immune system to fight it. I’ve experienced only very minor side effects, so it’s definitely worth it.
Another interesting aspect of the pharmacy bill is the relative cost of Keytruda vs. the chemotherapy drugs and infusions. Gemcitabine and carboplatin, both chemotherapy drugs, were billed at $648 and $542, respectively, per treatment. The anti-nausea infusion (fosaprepitant) was billed at over $2,200!
Keytruda’s list price is $10,474.08
Merck, the manufacturer of Keytruda, offers a co-pay assistance program that brings the out of pocket expense down to $0 for 65% of patients or up to $300 for 80% of patients. Unfortunately, I fall into the 20% that pays more than $300 per infusion. UCM applied for the program on my behalf, but the application was denied. I’ll need to follow up on this, as the program covers Keytruda plus “platinum chemotherapy and fluorouracil as the first treatment in patients whose cancer cannot be removed by surgery.” I’ve got the platinum chemo and the no surgery conditions covered, but apparently, not being on fluorouracil (5-FU) is the reason for the denial. I’ve taken 5-FU before (post radiation in 2018), but my side effects were very tough, so I’m glad I don’t have to take it again.
Merck Access Program
The response letter stated that the denial is because the “patient was nor prescribed the Program Product for an FDA-approved indication.” That isn’t surprising, since NPC is still a rare form of cancer in the US and there aren’t enough patients (i.e. limited potential market) to justify the costs of a drug trial to gain FDA approval. Most of the research studies into checkpoint inhibitor drugs for NPC is from Asia, based on drugs (carmelizumab and toripalimab) created and manufactured by Chinese companies.
I’m just glad that immunotherapy as a treatment option seem to be really effective. While my specific drug-condition combination is not as common, the very positive effects of immunotherapy drugs on other conditions gives me hope that my response will follow a similar pattern.